Part III: Standard State and Standard Electrode Potential Measurement
a) What is the standard state
What is standard state? This is the key to solving the problem. In fact, the choice of standard state is basically arbitrary. It is only chosen for practical convenience. Typically, the standard state of a monomer or compound is the pure substance at which the experiment is performed at a standard pressure (one atmosphere) and temperature (usually 25°C). In the previous example, when metallic silver and solid silver chloride are pure substances, they are in standard state.
On the other hand, the standard state of a substance dissolved in a solution (i.e., a solute) is the state in which it has an activity of 1, which is a unit that expresses the effective concentration of a solute (molar fraction, mass molar concentration, volume molar concentration, etc.). Next, we will use the familiar volume molar concentration.
In general, for any given chemical substance A, the chemical potential (chemical energy) can be expressed using Equation 12.
Here µoP is the standard chemical potential of a substance A. When the activity of A is 1, µA = µoP The chemical potential is temperature and pressure dependent. Under the most common thermodynamic conditions of constant temperature and pressure, the chemical potential determines the stability of substances, such as chemical species, compounds and solutions, and their tendency to undergo chemical reactions to form new substances, or to transform into new physical states, or to migrate from one spatial location to another.
On the other hand, activity can be expressed in terms of volume molar concentration, as shown in equation 13.
γA is the activity coefficient,Co is the standard concentration (1 mol dm-3)
When the concentration of chemical substance A is reduced to the limit, its activity factor is infinitely close to 1.
At this point, the interactions between solutes or between solutes and solvents become negligible and approach an ideal state. The standard state is the unit concentration of the chemical species assumed to be in this ideal state (the state of activity 1).
It may perhaps be a little difficult to understand, but the point is that the concentration of the solution should be adjusted so that the solute has an activity of 1.
(This is, of course, only possible if the activity coefficient of the substance is known in advance).
b) How to measure the standard electrode potential?
Thus, it seems that if one wants to obtain a standard electrode potential directly from an electromotive force measurement, it is sufficient to construct a cell2 such as the one below, a cell in which all reacting species are in a standardized state, and to measure its electromotive force.
Cu(s) | Pt(s) | H2(g, PH2 = P0) | H+(αq, α = 1 ), Cl-(αq, α = 1) | AgCl(s) | Ag(s) | Cu(s)
According to Nernst's formula, the electric potential of a Harned cell: can be expressed as Eq. 16
But in practice it is very difficult to assemble such a battery. It is necessary to prepare an aqueous solution of hydrochloric acid in which the activities of both hydrogen and chloride ions are 1. How should this be done?
Since it is not possible to measure the activity coefficients of individual ions, it is necessary to introduce the average activity coefficient γ± by converting Eq. 16 above into Eq. 17:
After substituting hydrogen ion concentration equal to chloride ion concentration equal to hydrochloric acid concentration and partial pressure of hydrogen equal to standard atmospheric pressure constant equal to 1, further transformations give Eq. 18.
In the infinite dilution state γ± → 1, the hydrochloric acid concentration CHCl is varied by various methods, and the electric potential, E, is measured each time, and the E obtained by extrapolating the electric potential to the point where CHCl → 0 is E0 (this corresponds to the definition of a return to the standard state of the solute).
In fact, the electric potential of the Harned cell is measured in this way, yielding the values listed in Eq. (11) earlier (Note 2). As in Fig. 5 it was created from the data in reference. [2]
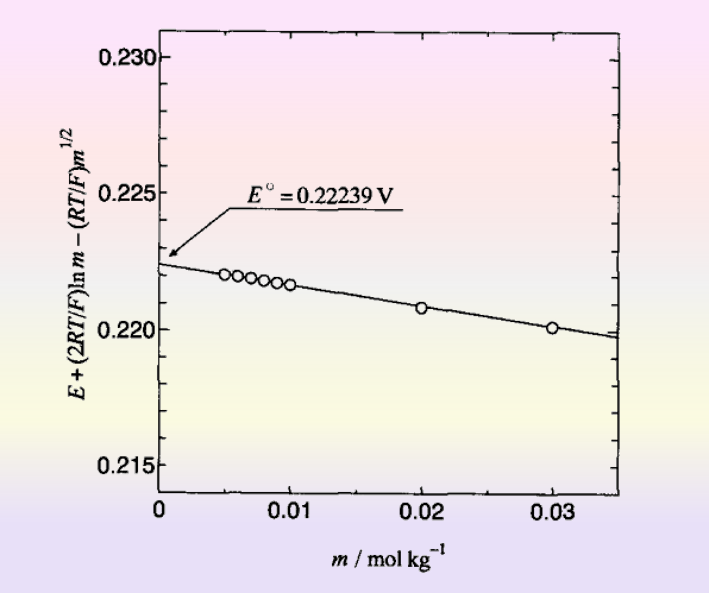
Fig. 5 Standard electrode potentials from electric potentials at infinite dilution.[2]
Many researchers have measured the standard electrode potentials of silver silver chloride electrodes. However, the numerical differences are of the order of microvolts. For details, see reference [3].
Thus, it seems that it is possible to obtain standard electrode potentials for various substances by measuring the electric potential at infinite dilution. However, this method cannot be used in all cases (very few systems can be measured). For example, the target reaction is very slow (since this is a problem for measuring the equilibrium potential), side reactions occur, surface films are formed, etc. In most cases it is not practical to measure. Even in such cases, the standard electrode potential can be obtained by a completely different method. In other words, a method that uses thermodynamic relationships.
Reference:
[2] H. S. Harned and R.W. Ehlers, J. Am. Chem. Soc., 54, 1350, (1932).
[3] R. G. Bates and J. B. Macaskill, Pure and Appl. Chem.,50, 1703, (1978).

