Part I: Review of Redox Reaction · Galvanic Cells and Electrolytic Cells
· Redox reaction
Redox reactions are chemical reactions in which electron gain or loss occurs.
We can split the redox reaction above into the following two half-reactions:
One is a reduction reaction where Ox1, the oxidized state of substance 1, gains electrons to reduce to Red1, the reduced state of substance 1 . The other is an oxidation reaction where the reduced state Red2 of substance 2 loses electrons and oxidizes to the oxidized state Ox2 of substance 2 Oxidation and Reduction reactions necessarily occur in pairs, and the half reactions cannot exist separately.
For redox reactions in which n electron transfers occur can be expressed in general terms as reduction half-reactions and oxidation half-reactions in Eqs. 2 and 3.
· Galvanic Cells and Electrolytic Cells
Primary cell is a device that converts chemical energy directly into electrical energy through a redox reaction, while an electrolytic cell is a device that converts electrical energy directly into chemical energy through an electrolysis reaction.
Electrolysis is the process of controlling the potential or current on the electrodes through an external power supply device, so that the two electrodes in the electrolyte solution have a potential difference (anode, cathode), and the oxidation-reduction reaction occurs respectively is called electrolysis.
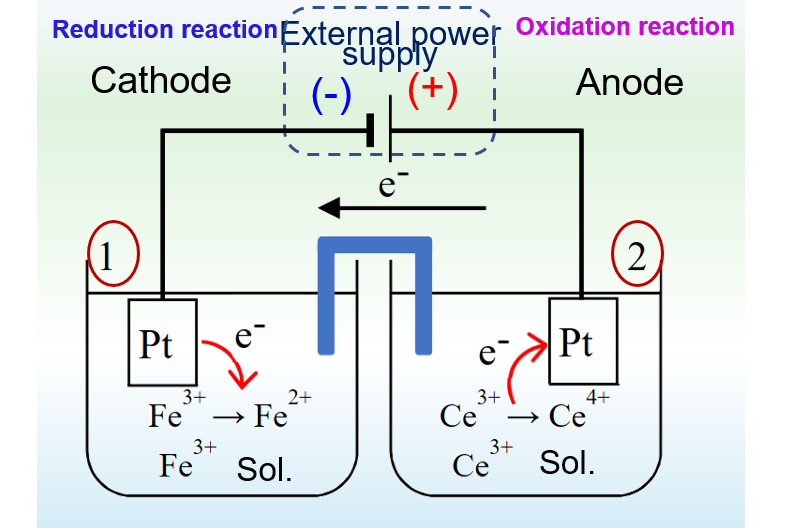
Here is a comparison of Figures 1 and 2 to further our understanding of primary cells and electrolytic cells.
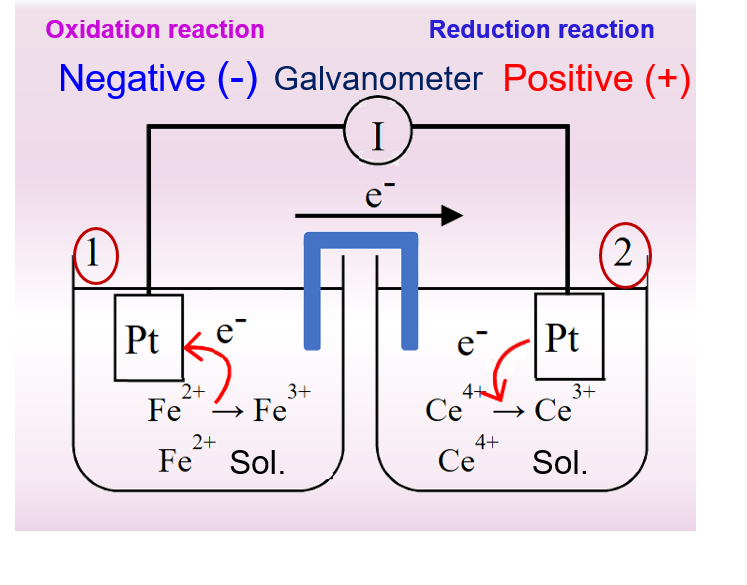
A primary cell consisting of an electric pair of iron ions and cerium ions is represented in Fig. 1 on the left. It can be seen that a spontaneous reduction reaction occurs at the positive electrode, i.e., the tetravalent cerium ion gains an electron and is reduced to a trivalent cerium ion, while an oxidation reaction occurs at the negative electrode, i.e., the Fe2+ ion loses an electron and is oxidized to a Fe3+ ion. Note that the two platinum electrodes in this primary cell do not participate in the electrode reaction only as a carrier for electron conduction.
For the electrolytic cell in Fig. 2, the electrode connected to the positive pole of the external power supply is the anode, where an oxidation reaction occurs and Ce3+ is oxidized to Ce4+. The electrode connected to the negative pole of the external power source is the cathode, where a reduction reaction occurs and Fe3+ ions are reduced to Fe2+ ions.
The electrode reactions in the primary cell of Fig. 1 and the electrolytic cell of Fig. 2 are inverse to each other. The primary cell works as a discharging process, while the electrolytic cell works as a charging process.
For the primary cell in Fig. 1, the overall cell reaction can be expressed as equation (4)
Following the convention to represent half-cell reactions, uniformly, in the form of reducing half-reactions, then the protocell reaction of Fig. 1 is composed of the two half-cell reactions of Eqs. (5) and (6).
If equation 6 is subtracted from equation 5, the electron e is eliminated, and then after shifting the terms, the same chemical reaction formula as equation 4 can be obtained. Similarly, the potential difference between the positive and negative terminals of the battery, that is, the electric potential of the battery, can be expressed as Right half-reaction potential (E2) minus Left half-reaction potential (E1)
The question then is, how is the electrode potential of the half-reaction obtained here? As mentioned earlier, the potential of the half-reaction, i.e. the potential of a single electrode, cannot be measured alone. Therefore, in practice, the potential of the reduced half-reaction can only be obtained by measuring the potential difference between the single electrode and the reference electrode (the electrode used as a reference).

