Part 13: Effect of supporting electrolytes
Since the cations and anions supporting the electrolyte react with species or products of opposite polarity, this can have various effects on the electrode reaction.
1) The reduction potential of a metal ion becomes more negative when the metal ion forms an ion pair or complex with the anion of the electrolyte salt.
2) The greater the cationic radius of the electrolyte salt, the easier it is to reduce the metal ion in the more strongly donor solvent (the reduction potential becomes more positive).
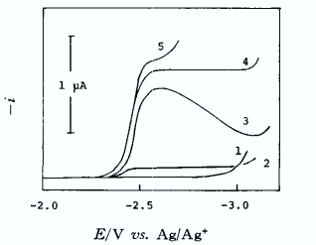
1, Me4N+, Et4 N+; 2, Bu4N+; 3, Hex4N+; 4, Hep4N+; 5, Li+ (0.05 M ClO4- salt each).
Na+ concentration was 0.5 mM.
When the supporting electrolyte cation is tetramethylammonium root, or tetraethylammonium root ion, the sodium ion is not reduced at all, and only a residual current is seen; when the supporting electrolyte cation is tetrabutylammonium root ion a very small reduction wave is obtained, which has the same characteristics as the reaction current.
When the radius of the electrolyte cation becomes further larger, as in the case of lithium ions, tetraheptylammonium root ions, etc., the reduction waveform of Na+ ions is close to a reversible diffusion-controlled waveform.
This phenomenon can be explained qualitatively as follows:
At negative potentials, cations with small radii are preferentially attracted closer to the electrode surface, whereas larger cations are prevented from approaching the surface by the presence of smaller cations. As the electrolyte cations become slightly larger, such as the tetrabutylammonium root ion, this effect becomes less pronounced, and some Na+ is reduced (by osmosis or desolvation) close to the electrode surface. When the cation becomes further larger, there is almost no interference effect and a diffusion-controlled rate reduction wave is obtained.
It can be seen that the effect of the cations of the supporting electrolyte on the reduction of metal ions at negative potentials is very significant in the most feeder (alkaline) HMPA, a phenomenon commonly seen in the reduction reactions of alkali and alkaline-earth metal ions in solvents such as DMSO, DMA, DMF, etc., which are highly feeder.
However, this phenomenon is less pronounced in weak donor solvents such as acetonitrile (AN) or propylene carbonate (PC).
3) Effect of cations supporting electrolytes on the reduction of organic compounds
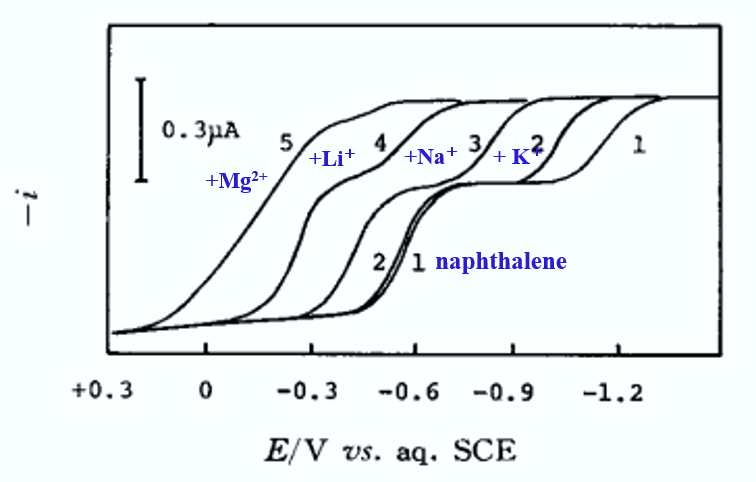
(1): 0.05 M Et4NClO4, 0.5 mM naphthoquinone, (2): (1) ten 5 mM K+, (3): (1) ten 5 mM Na+, (4): (1) ten 5 mM Li+, (5): (1) ten 5 mM Mg2+
In nonprotonic solvents, the anionic radicals of the first-step reduction products of the reduction reactions of organic compounds have the property of forming ion-pairs readily with various metal ions, so that metal salts can be used as the supporting electrolytes, or metal ions can be added to the 4-alkylammonium salt solution during the measurement of the polarograms, and, as shown in Fig. 9, the first reduction wave shifts to a positive potential (from the positive shift of the potential of this half-wave, the ion-pair generation constant can be derived).
It can be observed that the smaller the radius of the metal ion becomes, or the higher the charge number, or the smaller the donor solvent such as acetonitrile AN, or propylene carbonate (PC), the more likely it is that the half-wave potential will shift positively.
We know that the formation of such ion pairs promotes disproportionation and polymerization reactions of R∸ anion radicals (R∸). Therefore, sodium and lithium ion salts are not suitable as support electrolytes for measuring the reduction reactions of organic compounds.
Reactions at the second step where the anionic radical gains a further electron to generate a 2-valent alkyl anion will be more susceptible to supporting electrolyte cations than the first step reactions. The reason for the apparent positive reduction potential in the presence of alkali metal ions can be attributed to the fact that the 2-valent alkyl anion is more susceptible to ion-pair formation with the supporting electrolyte cation than the anion radical. On the contrary, when the radius of the 4-alkylammonium ions increases, the reduction potential gradually shifts toward the negative potential, and the reason for this seems to be that the effect due to the change in the structure of the double electric layer should be considered.
Reference
[17] K. Izutsu, S. Sakura and and T. Fujinaga, Bull. Chem. Soc., 45, 445 (1972); 46, 493, 2148 (1973).
[18] T. Fujinaga, K. Izutsu and T. Nomura, J. Electroanal. Chem., 29, 203 (1971).

