This is a basic content about the types of working electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
- Part 1: General Remark
- Part 2: Polarizable electrode and nonpolarizable electrode
- Part 3: Metal electrodes such as gold and platinum
- Part 4: Usually used working electrodes & features
- Part 5: Structure of GC & Electrochemical features of basal/edge plane
- Part 6: Glassy carbon electrode surface state characteristics
- Part 7: Glassy carbon electrode surface impurity adsorption and countermeasures
- Part 8: Adsorption of catechol and derivatives on glassy carbon electrode and electron transfer rate
4. Carbon electrode materials
Professor Noriyuki Watanabe
The physical quantity along the basal plane and the edge plane are anisotropy. The electrical resistance along the basal plane is smaller than along the edge plane. Thus, the electrode properties are different for basal plane and edge plane electrode surface (edge plane electrode has better conductivity).
Furthermore, the capacitance of the electric double layer on electrode surface are different, the electric double layer capacitance of the basal plane electrode surface is smaller.
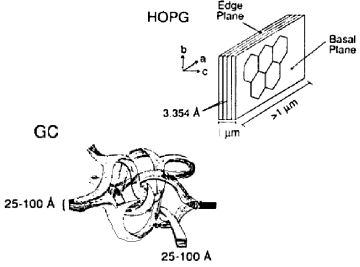
Fig.4-1 The schematics of Graphite and Glassy carbon structure.
• Pyrolytic graphite (PG) is prepared by decomposing the hydrocarbon gas at the high temperature substrate. Pyrolytic graphite further dealt with high temperature and high pressure could be enhanced in the crystalline ordering, and highly oriented pyrolytic graphite (HOPG) can be obtained. The electrode performance is depended on the crystalline orderly ratio State of electrode surface can be detected by redox peak potential difference (ΔEp) in cyclic voltammetry (CV) diagram of ferrocyanide/ferricyanide. If the redox peak potential difference is higher than 700 mV (1M KCl supporting electrolyte solution, potential scan rate 0.2V/s), this surface is regarded as the basal plane of the highly oriented pyrolytic graphite (HOPG) (the peak potential difference of commonly used graphite electrode is around 60 mV). It is known that faster the electron transfer is, smaller the peak potential difference (ΔEp) will be, because the electron transfer rate on basal plane of the highly oriented pyrolytic graphite (HOPG) surface is very slow, that is why the large ΔEp value is appeared.
• Glassy carbon (GC) is the most commonly used electrode material, the structure of glassy carbon is shown in Fig. 4-1 (the left lower schematics). A lot of graphite structure shaped thin strip intertwine with each other in glassy carbon, although the microscopic structure has orderly morphology, but the macro structure can be regarded as amorphous (glassy) carbon. Therefore, as the electrode materials, allotropes of carbon are sp2 carbon in graphite structure (excluding diamond electrode). The surface of glassy carbon electrode (GC) is the mixture of basal plane and edge plane. Glassy carbon (GC) is dense and hard as glass, without gas or liquid permeability. In contrast, highly oriented pyrolytic graphite (HOPG) has a slippery flexible structure along the edge plane, peeling along the basal plane, a fresh surface can be obtained.
• Carbon paste is obtained by dispersed graphite powder in oil to paste state , can be used as an electrode (carbon paste electrode).
• Carbon fiber used for electrode preparation is called carbon fiber electrode. Usually, carbon fiber is used for microelectrodes preparation. Embedding the carbon fiber into the plastic or glass, after cutting, a cross-section is used as a microelectrode. Including glassy carbon, the basic structure is sp2 carbon bond.
• Positive holes in valence band are produced and conductivity is generated when boron element is mixed into sp3 bonded diamond (so called as boron-doped diamond electrode. If nitrogen element is incorporated, electronic conductivity is occurred.). A boron-doped electrode especially has very wide potential window. It has chemical stability like diamond, and can be used for special applications.

