This is a basic content about the types of working electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
- Part 1: General Remark
- Part 2: Polarizable electrode and nonpolarizable electrode
- Part 3: Metal electrodes such as gold and platinum
- Part 4: Usually used working electrodes & features
- Part 5: Structure of GC & Electrochemical features of basal/edge plane
- Part 6: Glassy carbon electrode surface state characteristics
- Part 7: Glassy carbon electrode surface impurity adsorption and countermeasures
- Part 8: Adsorption of catechol and derivatives on glassy carbon electrode and electron transfer rate
1.General Remark
Professor Noriyuki Watanabe
We will describe some tips in using working electrode in following series of letter. It is not appropriate to distinguish which one is working electrode or counter electrode whenever we employ a system of two electrodes. In that case, it is just possible to discriminate them as anode or cathode. However, it is still unable to say exact values of potential of both anode and cathode, so that it remains uncertain whether purposed reaction is occurring or not. It is important to examine redox potential corresponding to intended reaction, for which the potential of working electrode should be measured.
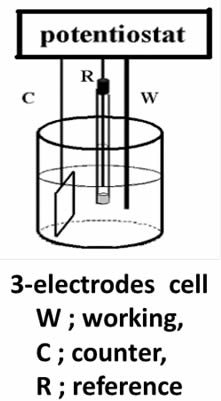
Distinct difference between working and counter electrode is applicable under using potentiostat. We can define the potential of a working electrode versus a reference electrode under so-called three electrode configuration (as shown in Figure below) by controlling with potentiostat. The understanding about potentiostat is desirable, even if not saying indispensable, which will be given later time.
We receive often the question that how the potential of counter electrode is going. It is quite reasonable question. The answer to this question is as follows. Amount of current flow in working electrode is exactly the same in counter electrode. The difference is just oxidative or reductive. If the current in working electrode is reductive (oxidative), the one in counter electrode should be oxidative (reductive). Hence, if depolarizing material (whatever it is) on counter electrode side without respect with either oxidative or reductive is scarce compared to the reaction of working electrode side, the overpotential in counter electrode side may glow exceedingly resulting in violation of compliance voltage. This difficulty can be mitigated by increasing the surface area of counter electrode in order to decrease current density. This is the reason why counter electrode with large surface area compared with working electrode is recommended, especially for large current application like bulk electrolysis.