TOP > Technical note > Basics for who are starting electrochemistry > Reference electrode
This is a basic content about the types of reference electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
A potentiostat is only to control an applied potential between working electrode and reference electrode as involving an undesirable voltage loss (i x Ru) caused by an unknown solution resistance Ru between them and a current i flowed through electrolysis cell. This resistance is called as uncompensated resistance, which gives an additional separation of cathodic and anodic peak potentials in cyclic voltammetry. As peak separation is also widen for irreversible electron transfer by relatively fast voltage scan. The discrimination of its origin could be sometime misleading. Potentiostat has a function to compensate the term due to i x Ru by an electric positive feedback in order to decrease this extra loss as less as possible, although not completely. It is better that reference electrode is located as close as possible to working electrode, in the extent of not to disturb the diffusion of analyte toward the working electrode. One way is to use a tube with narrow tip called Luggin capillary, in which reference electrode is inserted. It is important that the position of reference electrode should be fixed so as Ru not to be affected.
Although saying "let an electric current not flow into reference electrode", so far as reference electrode is united in electric circuit, minute current flows through it so that its impedance must be considered. An increase in impedance of reference electrode results in an increase of the time x constant, which is determined by double layer capacitance at interface of working electrode and impedance of reference electrode itself. It means that the band width of whole system including the electrochemical cell and potentiostat become narrow and its response gets slow. These situations often cause serious problem such as instability and fragility of potentiostat against to external noise. Precaution is required to avoid the further increase of impedance of the reference electrode caused by occurrence of precipitation in the frit.
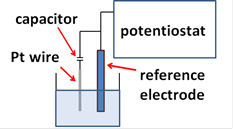
When the impedance of the reference electrode is large, the capacitor connected parallel to the reference electrode might be effective to avoid an oscillation of potentiostat (as like right Figure).
This is a basic content about the types of reference electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
8. Related subjects of reference electrode
Laboratory Of Research & Development, BAS Inc.
Professor Noriyuki Watanabe
Professor Noriyuki Watanabe


