This is a basic content about the types of reference electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
6. Reversible hydrogen electrode (RHE)
Professor Noriyuki Watanabe
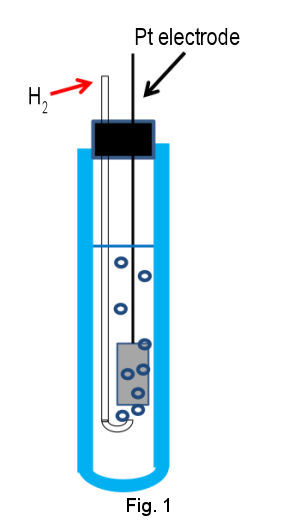
Primary standard of electrode potential is standard hydrogen electrode (SHE), which is composed of Pt electrode of high surface area immersed in acidic solution containing proton and hydrogen gas of standard activity (H+ and H2 of activity = 1) (Figure1 shown below). Pt electrode is utilized due to its high activity for redox reaction of hydrogen. The surface of Pt electrode is platinized in order to enhance the activity further by increasing surface area. To keep H2 content in steady state, hydrogen gas has to be continued to flow through internal solution by bubbling.
The electrode reaction and the electrode potential indicated by this electrode are shown below.
2H+ + 2e ⇄ H2 (1)
E = E0 + (RT/F) ln aH+ - (RT/2F) ln pH2 (2)
Taking E0 = 0 for granted is a consensus in the world of electrochemistry. Later two terms in the right side of eq (2) are both zero in standard state. Hence, E = 0 holds for standard state hydrogen electrode.
The electrode potential depends on proton concentration (activity, a H+) and hydrogen partial pressure (p H2) according to eq (2).
However, SHE is not convenient in practical use. That is a reason why the other electrodes already mentioned are employed for daily use. Even if using the other reference electrode, there are so many cases to report on SHE scale after converting experimental value to on SHE scale using well known difference. For example, in the case using SCE, SHE = SCE + 0.241 etc.
There is a convenient method to obtain approximate SHE during short time span, so called reversible hydrogen electrode (RHE), in other words, relative hydrogen electrode.
Commercial available one (RHEK), which is available by BAS Japan is utilized for temporary use. It basically consists of producing hydrogen gas in the reference electrode itself in every time of experiment by user. The evolved hydrogen on platinum electrode in strong acid solution (1.2 M HCl or 0.5 M H2SO4) by cathodic polarization is stored temporally in the reference electrode chamber. Although its purpose is a use in strong acid analyte solution, it is also usable in a neutral solution by joint use of double junction chamber. It is certified by the comparison with SCE and Ag-AgCl reference electrode that this RHE indicate approximately the same potential of SHE.
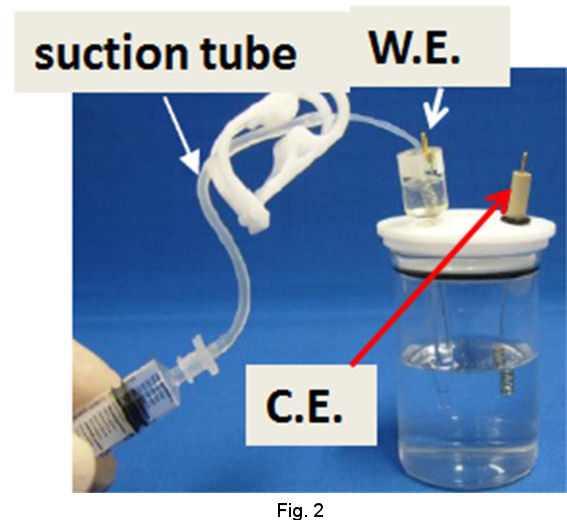
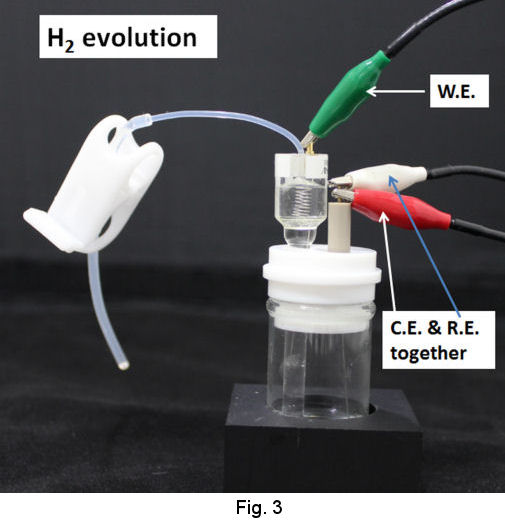
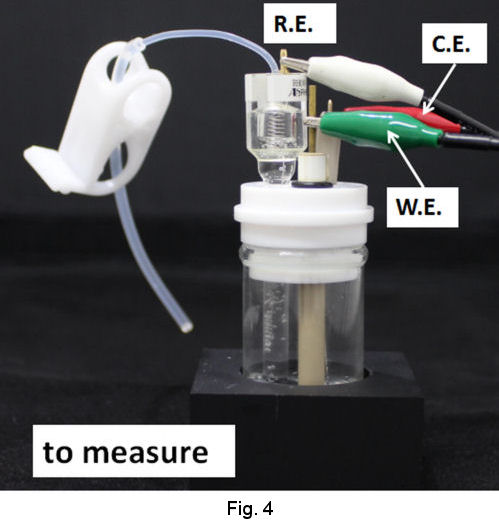
Operational procedures of RHEK are shown as follows. In the case of strong acid test solution, after siphoning up the strong acid solution into the tube of RHE main body (Fig. 2), let hydrogen gas evolve by cathodic electrolysis and store H2 in the RHE main body (Fig. 3). Then you can execute your primarily purpose after returning the working terminal which has been used on H2 evolution, to the working electrode in your test solution and exchanging the reference terminal which has been connected to counter electrode together with counter terminal(Fig. 4).
 |
 |
 |
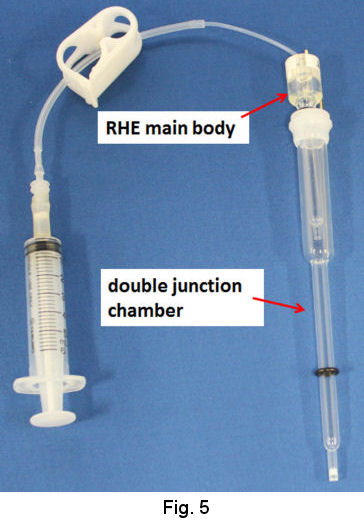
In the case of moderate acid and neutral solution or even strong acidic solution, use of double junction chamber is recommended (Fig. 5). The evolution of hydrogen gas is performed in the junction chamber by the procedure similar to that above mentioned.
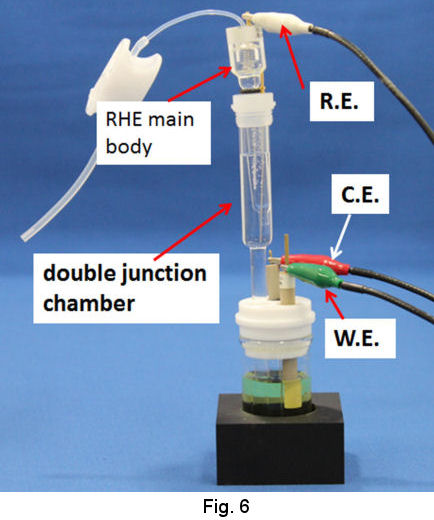
As the junction chamber has a frit on its tip, the junction itself can be removed from the strong acidic solution in order to be transferred to your test solution. Then, the RHE combined with the junction is used as ordinal reference electrode (Fig. 6).