This is a basic content about the types of reference electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
3. Calomel electrode
Professor Noriyuki Watanabe
Equilibrium reaction shown below takes place in calomel electrode.
Hg + Cl- ⇔ 1/2Hg2Cl2 + e- (1)
The electrode potential is given as following equation.
E = E0Hg22+/Hg + RT/2F ln Ks(Hg2Cl2) - RT/F ln aCl- (2)
Early two terms in right side of equation (2) is replaced to E0Hg2Cl2/Hg which is calculated to be 0.273V from the literature values of redox potential
(E0Hg22+/Hg = 0.7960V) and the solubility product constant (Ks(Hg2Cl2)=2×10-18).
Replacing again E0Hg2Cl2/Hg to E0, the equation (3) is obtained, which is familiar formula involving the activity of chloride ion.
E = E0 - (RT/F) ln aCl- (3)
Although a small discrepancy in above calculated value exists, E0 is known as 0.268 V for standard potential at 25°C. The electrode potential depends on the concentration of chloride ion as like silver-silver chloride electrode. It is 0.280 V at 1M KCl and 0.241 V at saturated KCl which is so often called as SCE as abbreviation of Saturated Calomel Electrode.
SCE has been used as reference electrode instead of SHE, since early days of electrochemical reserach. However, its use has been put the brakes by concern coming from environmental issues. However, still SCE remains at an important and indispensable position. As a matter of fact, we are able to see many recent reserach papers in which SCE is used. A typical structure of SCE is shown in right figure.
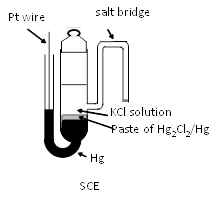
The reference potential of SCE is 0.241 V(at 25°C) , because of increased activity of chloride ion in internal solution of saturated KCl (ca. 4.8 M). In addition to the chloride concentration dependence of potential, the temperature dependence of potential is about -0.5 mV per degree. Except for SCE, the reference potentials are 0.280 V and 0.334 V for 1 M KCl and 0.1 M KCl, respectively. The higher the concentration of KCl is, the larger the temperature dependence of the potential is.
The side arm as salt bridge extended from the body of SCE in above figure is not convenient for daily use because of its fragility. Hence, mercury electrode is placed uppermost and a paste of mercury-mercurous chloride is placed under it and supported by ceramic etc. Such type of SCE electrode is available from BAS Inc. (RE-2BP as shown right Figure).

We often receive the question of "Is SCE usable for experiment in organic solvent?" . Answer is "Yes". However, in the case when you dislike a contamination with water or chloride ion, you are recommended to use a salt bridge, in which both the same organic solvent and electrolyte used in your test solution is contained. Even so, you have to pay attention the fact that a rather large junction potential between SCE and the salt bridge still remains unknown. In addition to that, considering a growing risk of deposition in the frit, it might be better to use a reference electrode for non-aqueous solvent(such as Ag-Ag+ in organic solvent ) or pseudo-reference electrode (such as Pt wire), then refer to the internal standard such as ferrocene.
Usage of pseudo-reference just connecting to the reference terminal of potentiostat is recommendable from the stand point of low impedance.

