The topics are listed below:
2. Silver-silver chloride electrode (SSCE)
Professor Noriyuki Watanabe
A reference electrode is used as in equilibrium so that the net-current in circuit connected to the reference electrode is desired to be zero. Or rather it must be used under zero current. Connecting terminal for reference electrode in potentiostat has extremely high input impedance so that it is impossible for current to flow through the reference electrode. That is one of the important functions of potentiostat.
The most used reference electrode nowadays is silver-silver chloride electrode (SSCE). SSCE is environmentally friendly compared to calomel electrode using mercury and its salt. Its stability, reliability and reproducibility are also sufficient.
The reaction on SSCE is shown as below.
Ag + Cl- ⇔ AgCl + e- (+0.20V)
Forward reaction is oxidation and reverse is reduction. These reactions are in equilibrium.
Chloride ion (Cl-) comes from internal electrolyte (KCl or NaCl). Ag is silver wire itself. AgCl is thin solid film formed on surface of the silver wire.
It is easy to manufacture Ag/AgCl electrode. It is just anodizing cleaned silver wire in water solution containing chloride ion (KCl or HCl etc.). The film formed on surface of the wire is faint pink and turns to dusky grey with time. Thus obtained wire is put into KCl solution confined in a glass tube with a frit in its end for electric contact with test solution as shown in right Figure. That’s all.
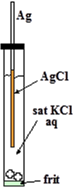
The electrode potential of above reaction is given as follows.
E = E0Ag+ + (RT/F)ln aAg+ eq.(1)
Substitution of the activity of silver ion with the solubility product constant of AgCl , KsAgCl results eq(1) into following equation (2).
E = E0Ag+ + (RT/F) ln KsAgCl - (RT/F) ln aCl- eq.(2)
Using literature values of E0Ag+ and KsAgCl ( E0Ag+=0.7991 V, and KsAgCl = [Ag+][Cl-] = 1.6×10-10), well-known equation (3) is obtained.
E = 0.222 - (RT/F) ln aCl- eq.(3)
aCl- is the activity of chloride ion in internal solution of the electrode. So, the reference potential of SSCE depends on the activity of chloride ion in addition to temperature. An increase of the activity results in a cathodic shift of the potential. Hence, keeping internal chloride concentration to constant is important to obtain reproducible results.
Reference potential of saturated SSCE(in saturated KCl solution) is 0.197 V at 25°C versus standard hydrogen electrode(SHE). Electric contact of reference electrode with test solution is performed through the frit which permits mutual transfer between the internal solution and the test solution. Hence, dilution of the internal solution and contamination of the test solution provides a possible significant trouble sometimes.
Instead of KCl, sometimes NaCl is chosen as an internal electrolyte. When perchlorate anion is involved in test solution, NaCl is often employed as internal electrolyte because of the low solubility of potassium perchlorate compared with sodium salt. Deposition of potassium perchlorate within the frit may cause serious trouble like large increase of reference electrode impedance.
A main reason of using KCl is that potassium cation and chloride anion have almost identical mobility so that the liquid junction potential at the liquid interface composed of these ions is minimized. While, the liquid junction potential for the case using NaCl as internal electrolyte may become rather considerable. Which one is better choice might depend on a compromise among practical convenience.
Management of reference electrode is important, otherwise resulting in shift of reference potential. Reference electrode should be kept in solution of the same composition of internal solution, while it is not used. Contamination or deposition within the frit due to external substances should be excluded, otherwise resulting in big trouble such as oscillation of potentiostat caused by external noise etc.
Check method of Ag/AgCl reference electrode
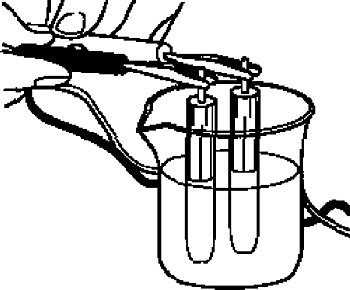
For the check, if the reference electrode is properly, you can measure comparing with the new one of the same type.
Since the reference electrode in the cell is the same, the ideal potential difference is 0 Volt, however in a practice a small potential difference will occur.
It can be used if the potential difference, between the electrodes, is within 0 ± 20 mV.
For the measurement, immerse the reference electrode in 3 M NaCl solution or saturated KCl solution, and then measure the potential difference between both them using a voltmeter.
Reference: Potential for difference type of the reference electrode.
NHE(Normal Hydrogen Electrode)・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・0mV
SCE(Saturated Calomel Electrode)・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・241mV
SSCE(Sodium Saturated Calomel Electrode)・・・・・・・・・・・・・・・・・・・・・236mV
Ag/AgCl(SaturatedNaCl)・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・201mV
Ag/AgCl(Saturated KCl)・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・198mV
Hg/HgSO4(Saturated HgSO4)・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・616mV
Cu/CuSO4(Saturated CuSO4)・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・300mV

