This is a basic content about the types of reference electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
1. Introduction
Professor Noriyuki Watanabe
In electrochemistry, a specific potential is applied upon a working electrode in order to let a specific reaction occur. The potential of a working electrode should be always exactly specified. That is the reason requiring a reference electrode as reference of the potential.
The reference electrode consists of:
- metal electrode (platinum, gold, mercury, silver, carbon etc.)
- internal electrolyte solution
- a frit (ceramic, Vycor etc.).
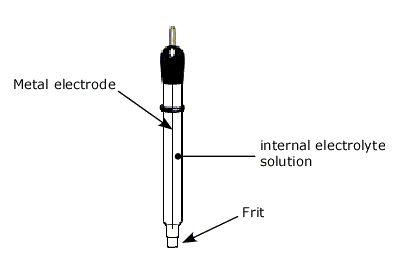
Typically a small glass tube (sometimes tubular Teflon for anti-corrosion) is used as the container for these three components.
The frit is located at the end of the tube for electric contact with test solution. Requirement of a reference electrode is a long time stability in constant indication of potential. For that, electric current should not be allowed to flow through the reference electrode. Since the current flow may cause a drift of potential, a reference electrode is desired to be connected to a high impedance circuit. Potentiostat has a reference electrode connecting terminal which is high input-impedance terminal. Hence, current flow is strictly limited through a reference electrode.
Although only connecting a platinum wire, which is immersed in a test solution, to the reference terminal of potentiostat makes electric contact with the test solution, so called pseudo-reference electrode, but that could not guarantee the stability or reproducibility of potential measurement. It might be worthy to note that reproducible measurement is possible only by connecting a reliable reference electrode.
Although electric current does not flow through reference electrode, internal impedance of reference electrode itself is better as small as possible. As potentiostat is an automatic control system, involving a high impedance component in the feedback loop results in decrease of response rate (in another words, narrowing of band-width), then it destabilizes to oscillation. It is necessary to avoid the formation of deposit in the frit located in the end of reference electrode, which might result in extremely high impedance of the reference electrode.
Standard hydrogen electrode (SHE) is a primary standard of potential, but it is not necessarily convenient for daily use because of troublesome handling of hydrogen gas. Instead of SHE, as secondary reference electrodes, saturated calomel electrode (SCE), silver-silver chloride electrode (SSCE) and reversible hydrogen electrode (RHE) etc are well known and used.
Why does a reference electrode indicate a constant potential ?
The reason is that a redox reaction on the electrode surface occurs reversibly and equilibrates all the time as shown below.
SHE H2 ⇔ 2H+ + 2e- (0V)
SCE Hg + Cl- ⇔ 1/2Hg2Cl2 + e- (+0.24V)
SSCE Ag + Cl- ⇔ AgCl + e- (+0.20V)
The electrode, which is able to consist of a redox pair in equilibrium is called a reference electrode. The potential of a reference electrode is not able to be changed externally so that it is called non-polarizable electrode. On the contrary, platinum, gold and carbon, of which potential are able to be changed externally are called polarizable electrodes and are used as working or counter (auxiliary) electrodes.
As the relative potentials among the various reference electrodes are fixed and well-known, it is of significant meaning that the comparison between experimental data obtained in even if different places and times is able if the reference electrode used is specified.

