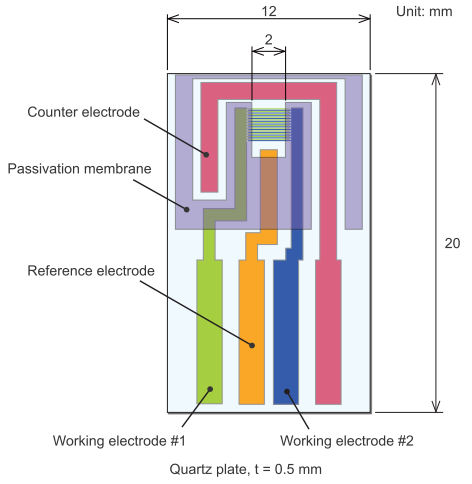
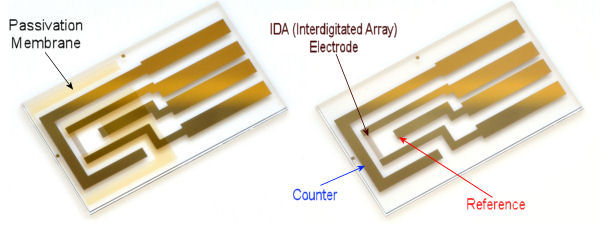
IDA (Interdigitated Array) Electrode
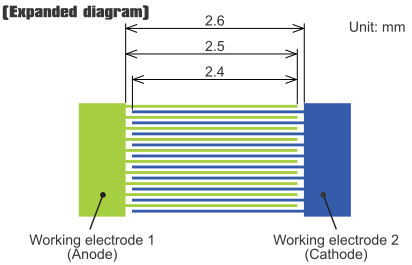
Cyclic Voltammetry Measurement using IDA Electrode
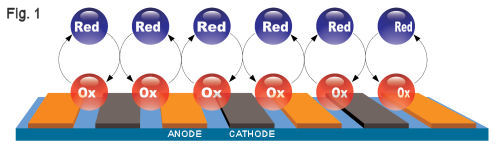
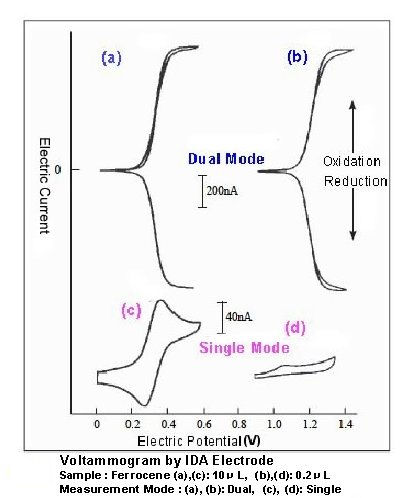
Fig.3 shows voltammograms of ferrocene samples ((a),(c): 10 uL , (b),(d): 0.2 µL) using IDA electrode ((a),(b): Dual mode; (c),(d): Single Mode). As they indicate, measuring mode makes obvious difference on their responses. Dual mode reinforces reduction current at collector electrodes with increase of oxidation current at generator electrodes. At the measurement (d), response is scarcely obtained because the sample was consumed during the experiment.
IDA (Interdigitated Array) Electrode 3 µm
More fine IDA (Pt/Au) Electrode has been developed. Its 3 µm pitch working electrodes significantly increase redox cycle and allow
researchers to perform ultra-sensitive measurement.
IDA Au (3 µm)
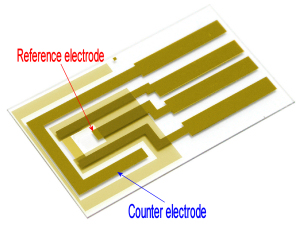
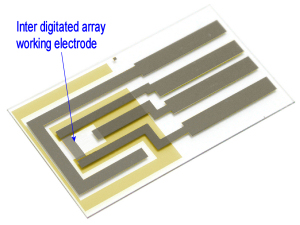
We recommend to apply Ag/AgCl ink to a reference electrode in order to stabilize potential of the electrode.
Micro Volume Sample Measurement with IDA Electrode 3 µm and Cable kit
- Insert IDA Electrode 3 µm onto Cable kit
This electrode can be used with Cable kit for IDA electrode (Catalog No.011066).
- Put sample solution by dropping on the IDA electrode
- Make the solution to cover comb-shaped working electrode, reference electrode and counter electrode
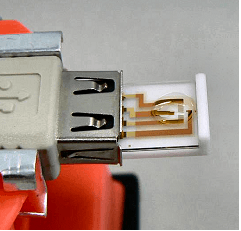
- Researchers can comfortably measure by using a Dual electrochemical analyzer such as ALS800B series and 700C series.
Technical data concerning IDA Electrode 3 µm
Fig. 1 -- Accuracy
A CV curve obtained by 3 µm IDA Electrode Au [A] mirrored that of 3 µm IDA Electrode Au [B], which means both of the IDA Electrodes were produced very accurately.


- Left: 3 µm IDA Electrode Au [A]; Right: 3 µm IDA Electrode Au [B]
- Sample: ferrocene methanol (1 mM/ 0.5 M NaCl solution)
- Mode: Single
- Sweep Rate: 30 mV/s
Fig. 2 -- CV by Dual mode
- Mode: Dual
- Sweep Rate at G-Electrodes: 10 mV/s
- Applied Voltage to C-Electrodes: -0.2 V
Fig. 3 & 4 -- Comparison of 3 µm IDA Au with 10 µm by Dual Mode
For 3 µm electrode, the limiting current of ferrocene methanol by CV Dual mode was 8 times as much as of the peak current by Single mode, while this ratio for 10 µm electrode was about 4. This means large increase of the redox cycle.
Fig. 5 -- Capture Efficiency
Figure 5 shows a result of LSV Dual mode measurement. Its collection efficiency was proved to be 96.4% by our calculation from limiting current of Collector electrodes and Generator electrodes.
- Method: LSV (Linear Sweep Voltammetry)
- Mode: Dual
- Sample: ferrocene methanol (1 mM/0.5 M NaCl solution)
- Sweep Rate: 10 mV/s
Besides CV and LSV electrochemical techniques, our IDA electrode is available for various electrochemical measuring techniques such as chronoamperometry, normal pulse voltammetry, differential pulse voltammetry etc.
Researchers can obtain more insights by using IDA electrodes.
IDA (Interdigitated Array) Electrode - 2 µm
More fine IDA (Pt/Au) Electrode has been developed. Its 2 µm pitch working electrodes significantly increase redox cycle and allow
researchers to perform high-ultra-sensitive measurement.
Technical data concerning IDA Electrode 2 µm
A CV curve obtained by 2 µm IDA Electrode Au.
CV by Single mode
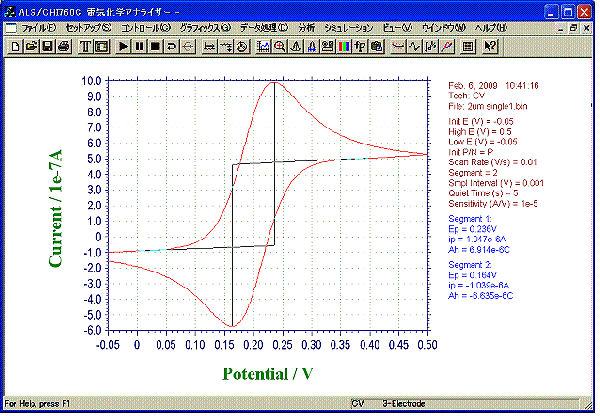
- Sample: 1 mM ferrocene methanol (0.5 M NaCl solution)
- Mode: Single
- Sweep Rate: 10 mV/s
CV by Dual mode
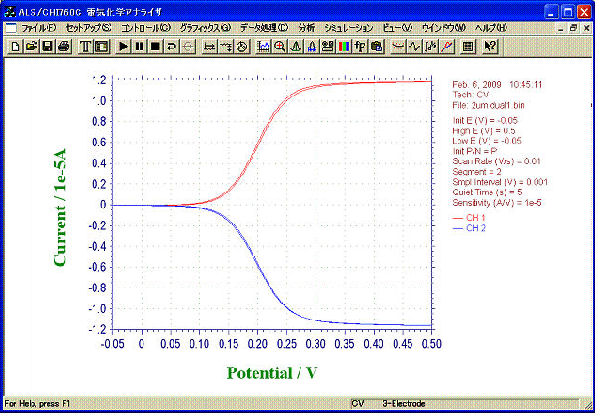
- Sample: 1 mM ferrocene methanol (0.5 M NaCl solution)
- Mode: Dual
- Sweep Rate at G-Electrodes: 10 mV/s
- Applied Voltage to C-Electrodes: -0.05 V
Its collection efficiency was proved to be 97% by our calculation from limiting current of Collector electrodes and Generator electrodes.
Capture Efficiency
Compared with IDA Au 10 µm and 3 µm, the capture efficiency of the 2 µm is higher, 93% for 10 µm and 95% for 3 µm.
For 2 µm electrode, the limiting current of ferrocene
methanol by CV Dual mode was 11 times as much as of the peak current by
Single mode, while this ratio for 10 µm and 3 µm electrode were about 4 and 7.5, respectively. This means large increase of the redox cycle.
Handling precautions of IDA electrode
The IDA electrode is a very sensitive product.
Here, some advice for the preservation and handling of IDA electrode will be described.
Attention for receiving the product
After receiving the product, it is recommended to test the insulation between the working electrodes using a tester.
Attention for opening the package
- When you will take out the electrode from the package, catch with the fingers at the edge of the glass substrate or pinch the glass plate carefully with tweezers. Glass substrate could break easily, so keep away from excessive force and strain.
- Do not touch the pattern area of the electrode directly. If you touch the pattern area, it could be peeled or break out.
Attention for cleaning before use
- Do not clean in ultrasonic cleaner.
- Do not clean the electrode with strong acid or base solution.
- Do not clean in ozone cleaner.
- Do not scratch the electrode surface to avoid the pattern area conductivity break down.
- If you want to clean the electrode with organic solvent, only rinse with acetone or ethanol. Do not immerse the electrode in an organic solvent for long time to avoid peeling of the electrode pattern and passivation membrane.
Attention in measurement
- The electrode can not be used in high and low temperature.
- Do not use the electrode in strong acid or base solution.
- Do not apply excessive oxidation or reduction potential.
- Physical or chemical modification of the electrode will be for your own responsibility. We do not guarantee the electrode characteristics after modification.
- This electrode is assumed as disposable. Reuse of the electrode is not recommended.
Attention in storage
If long-term storage, put the electrode in the case. Store the case in clean space such as desiccator, away from heat and moisture.
CAUTION
Any defects in the appearance (ex: pinhole on lead area, burr of glass) without any influence to the measurement, are not covered under warranty.





