Redox potential of molecule under research have to fall in the potential window over which the background current due to the electrode itself must not disturb the experiment.
Pt (Platinum) and Au (Gold) electrodes - applied to organic or inorganic substances measurement because they have high overpotential for oxygen evolution and low overpotential for hydrogen evolution.
Hg (Mercury) electrode - well suited to measure reduction of metal ions because of its high overpotential for hydrogen evolution. Hg electrode can easily observe the reduction of Zn.
At the measurement with non-aqueous solvent, hydrogen/oxygen overpotential does not affect. Instead of that, decomposition potential of the non-aqueous solvent and supporting electrolyte comes to an important factor. Furthermore, impurity of water in non-aqueous solvent makes the potential window narrower depending on the quantity of the water.
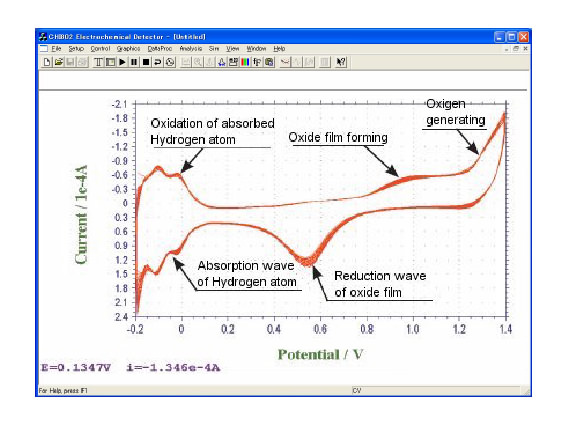
Figure: Current-Potential curbs of the result of 50 times scanning by immersing a Pt electrode in 0.5 M H2SO4 solution. Reiteration of the cycle obviously activates the electrode. Although there is absorption wave at the cathode side, it is possible to observe other reaction of the electrodes in this potential range because this reaction requires only certain quantity of electricity.

