ITO (Indium Tin Oxide) Transparent Electrode
#refx(): File not found: "ito_electrode.jpg" at dir "xdata/"
Working, Reference, counter electrode all combined ITO electrode
ITO electrode is a transparent electrode generally used for Spectroelectrochemical measurement. ITO is an abbreviation of Indium Tin Oxide, printed to quartz plate to form electrode by vacuum evaporation method. ITO layer thickness is 100nm, have about 20 ohm electric resistance. ITO transmit light in visible range.
Also provide an ITO electrode in other design to fit your experiment. Please contact us for a special order of ITO electrode,
In addition to ITO vacuum evaporated type flat electrode, we also producing a printed electrode special order as ring disc shape electrodes, split disc shape electrodes and interdigitated array electrodes.
ITO electrode optical characteristic

ITO electrode spectrum background data
As figure showed above, ITO show a spectral absorption in ultra violet range.
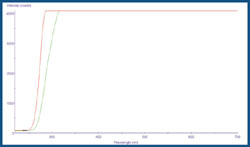
Absorption spectrum of ITO electrode and quartz glass'' (light transmiting intensity)
ITO electrode is a unique transparent electrode material. WE can also prepare special order cell by users demand.
Spectroelectrochemical Cell Electrode
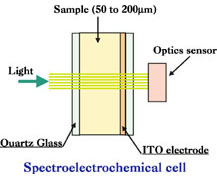
Figure below show the structure of ITO spectroelectrochemical cell. The part which transfer light are the ITO electrode part. A measurement can be performed using fiber optic spectrometer, light source and fiber optic to transmit light with a proper wave length through electrode. Absorption take place to proper light range which pass electrode due to reaction occur on electrode and detected by spectrometer.
Applications :
- For ion transfer analysis on liquid/electrode surface
- Spectroscopic analysis on electrode surface or near surface
- Spectral absorption analysis of product or intermediate
- Reaction parameter analysis as concentration, duffusion coefficient, intermediate life span, etc.
Pt and ITO are vacuum evaporated onto quartz plate. ITO is transparent electrode so it can transmit light. Pt electrodes used as counter and reference electrode.
3,3'- Dimethylbenzidine (o-Tolidine) used as measurement sample to generate chemical reaction as showed below.
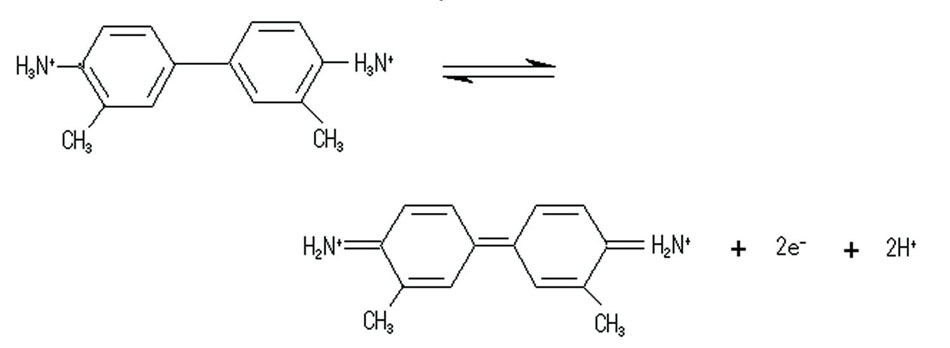
Using thin layer ITO cell, 25µm gasket between ITO electrode and glass cover to perform CV measurement and to observe spectral change take place in reaction simultaneously.
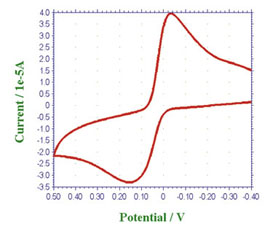
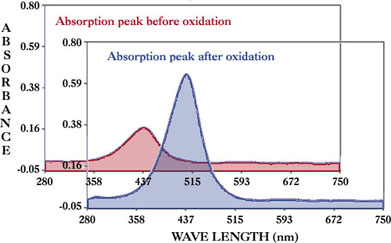
Absorption intensity change occur on redox reaction at CV measurement (50mV/s) can be observed clearly.
For a Spectroelectrochemically active sample, a reaction will cause a special change in spectrum and electrochemical property, and the reaction mechanism can be studied more detail by performing a simultant analysis.
Measurement conduct using fiber optic spectrometers from OOI (Ocean Optics) Inc. and an electrochemical potentiostat.

